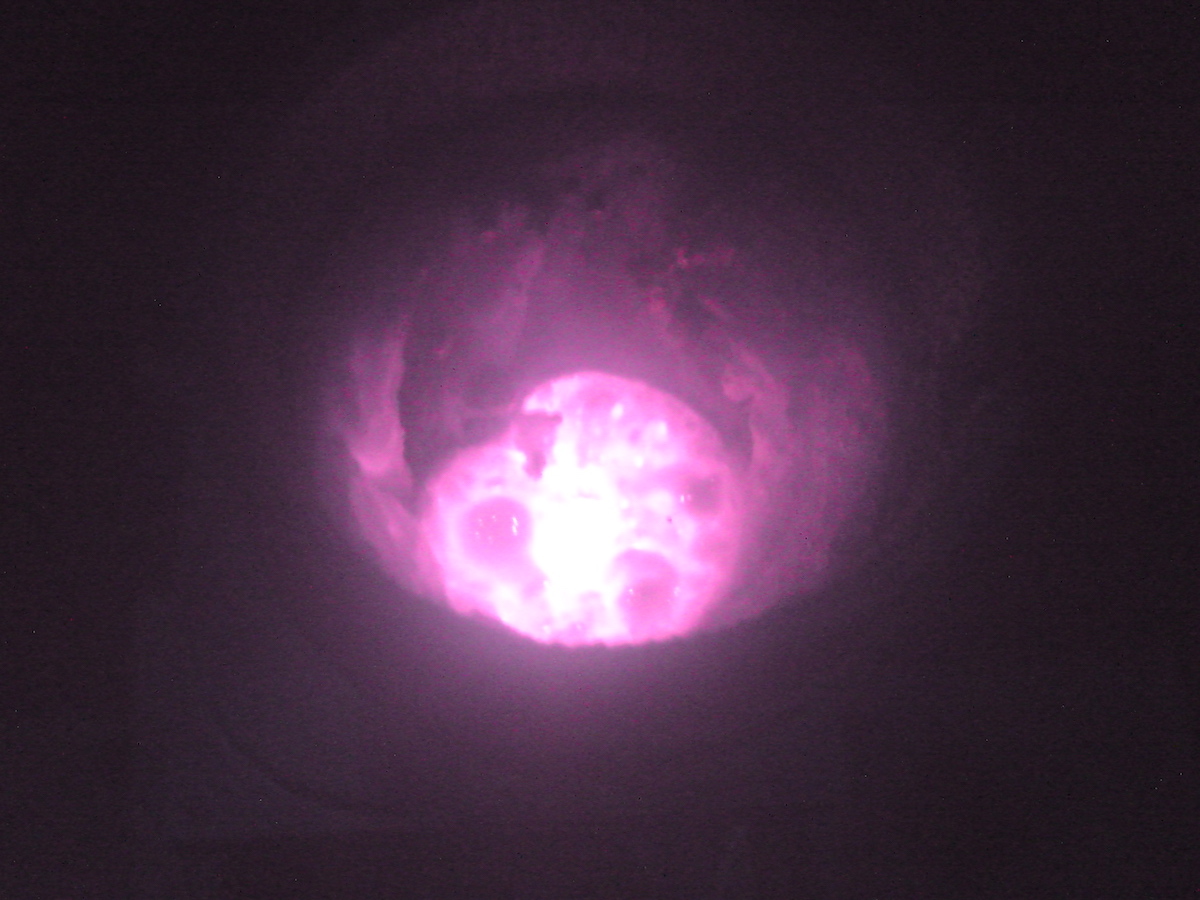
The thermite reaction
The thermite
reaction is very useful, and very fun. I've seen it used for
spot welding during the construction of our local tram here in Dublin, and
I've also seen it explode powerfully late at night in a remote part of the
Dublin mountains.
Thermite reactions are classic examples of oxidation / reduction. One metal
is burned and another is reduced to its elemental state. The traditional
reaction is:
$$Fe_2O_3 + 2Al \to 2Fe + Al_2O_3.$$
In early 2009 I gathered a few friends and made a couple of trips up the
Dublin mountains where we carefully experimented with thermite. We tried
each of iron, copper, and manganese thermites and we successfully ignited
them with a magnesium fuse, as well as via the
glycerol and potassium permanganate
reaction.
Safety first
The intention of these trips was to have a little fun, in the spirit of
fireworks, but they also provided
a valuable unexpected lesson: safety first. We were very
careful and I recall thinking we might have been taking ourselves a bit too
seriously. As it turns out the copper thermite reactions exploded
powerfully, rather than burning as expected.
Fortunately all was well as we always ensured we were far away at
ignition time. We watched from a distance as it literally rained molten copper.
Some videos
Copper thermite explostion (video)
Standard iron thermite reaction (video)
Unfortunately I had to mute these for the sake of others' privacy so you
cannot enjoy the satisfying boom.
Some photos